
QS-Report:
Fruit, Vegetables, Potatoes | issue September/2017
3
Be it audit or monitoring data – quality
assurance within the QS scheme relies on
detailed information. QS therefore uses
state of the art IT technology in the service
of its scheme participants for the col-
lection, storage and processing of this
data. The security and reliability of this
technology is equally as important as
the trustworthy handling of the data
and information. After all, it all provides
information about the product range and
the efficiency of the companies concerned.
There are more than 30,000 scheme
participants, 96 coordinators, 76 labora-
Data security and usage
Scheme participants can depend on data protection policy
QS Laboratory Performance Assessment
Constantly growing challenges create added value
tasks within the QS scheme. Passing of the
information to third parties is not permitted,
unless the participant concerned has
expressly consented otherwise.
(3)
All QS scheme participants have
permanent access to all basic, audit and
monitoring data that is saved within the
QS scheme relating to their company.
However, this does not include implicit
requirements of data protection which
have always been respected as part of the
QS scheme but are now consistently being
implemented in agreements and contracts
and as part of IT technology.
toriesand22certificationbodieswith216auditorsworkingtogetherin
the QS scheme for Fruit, Vegetables and P otatoes. The daily handling
of data requires clear rules for the collection, processing and use of
data. QS scheme participants can fully trust the data security within
the QS scheme. Taking account of the data protection regulations, QS
has derived three principles for the use of the data in the QS scheme.
(1)
The data of the scheme participants is only used for quality
assurance within the QS scheme. The use of the information for
other purposes is out of the question, unless the participant
concerned has expressly consented otherwise.
(2)
The data will be used by QS. Coordinators, scheme participants,
certification bodies, auditors and laboratories may only use the
information to the extent required for the performance of their
Dr. Gustav Offenbächer
Specialist in residue analysis
“
The test design for the Laboratory Performance Assessment is
being continually developed and adapted to incorporate current
industry issues. The permanent modification of the evaluation
criteria and test design selectively expose deficiencies in the analytical procedures
of the laboratories. This means the laboratories are constantly made aware of critical
analytical issues and thus can continuously improve the quality of their analyses.
”
Introduction of
an annual meeting for
laboratory managers
of the participating
laboratories for the processing of results from
previous laboratory performance assessments.
Introduction of
several test
materials with different active
ingredients. The number of
active substances as well as
their content vary. Processing
time for the sample is reduced
from 10 to 5 days.
Evaluation amended
:
no statistical z-score evaluation
any more, but 70-120% of the
amount of the active ingredient
added must be quantified.
Single method analysis
becomes
an integral part of the laboratory
competence assessment.
Point
system is overhauled
, so that
laboratories scoring below a
minimum number are even more
so obligated to participate in the
subsequent test once again.
Additional focus
on metabolite
analysis
. In addition to the parent
substance, degradation products
or metabolites must also be
considered in the analysis.
Test matrix no longer announced
in advance. Sample processing
time is reduced further.
Introduction of a points system
on
the basis of which the participation
of laboratories in the performance
assessment is evaluated.
Laboratories must send the original reports
to QS in addition to the delivery of their
test results. They are important for the
interpretation of results.
Shipping period for the sample
specified rather than a fixed
delivery time. Successive
adjustment of sample processing
time
from five days to four.
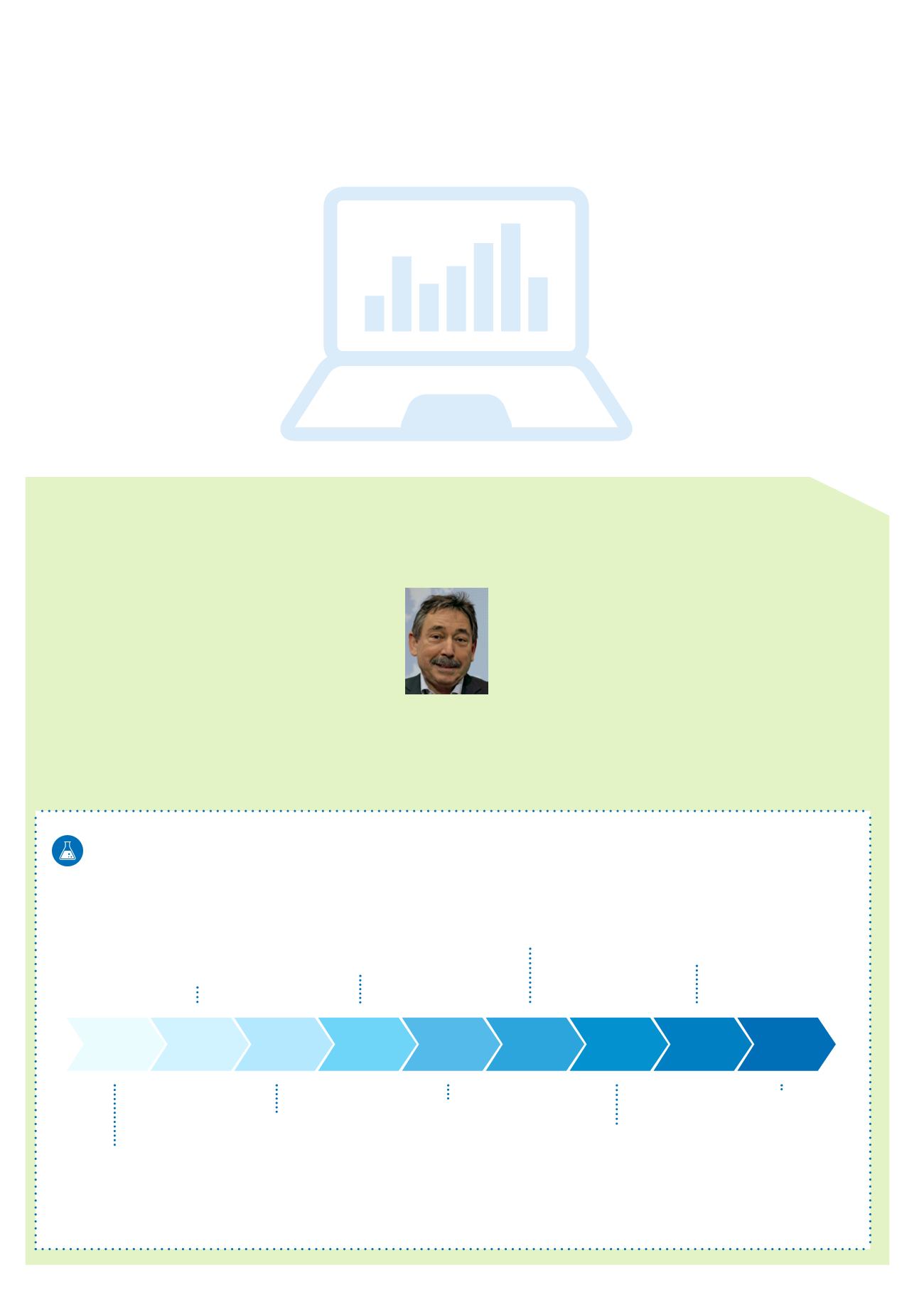
First QS Labora-
tory Performance
Assessment
August
2006
November
2006
2009
Autumn-test
2011
Spring-test
2015
Autumn-test
2007
Autumn-test
2010
Spring-test
2014
Autumn-test
2016
Spring-test
QS Laboratory Performance Assessment
Progression from 2006 to 2016
Twice a year, the analytical quality of accredited laboratories is
bench-tested by using the QS Laboratory Performance Assessment.
In the analysis of residues in fruit and vegetables, the qualification
of laboratory staff is particularly crucial, along with the equipment
and experience of the laboratory. Since the first competency tests
in 2006, the test design has been regularly modified and always
presents the laboratories with new challenges. An approach that
establishes reliability for all market participants but which also pays
off for the laboratories. They receive feedback with important in-
sights for the continuous improvement of their analytical processes.
















